Do you search for 'activity series of metals essay'? You will find all of the details here.
Table of contents
- Activity series of metals essay in 2021
- Reactivity series of metals
- Activity series worksheet
- Reactivity of metals
- Activity series chemistry
- Lightest metal
- Properties of metals
- What is the activity series
Activity series of metals essay in 2021
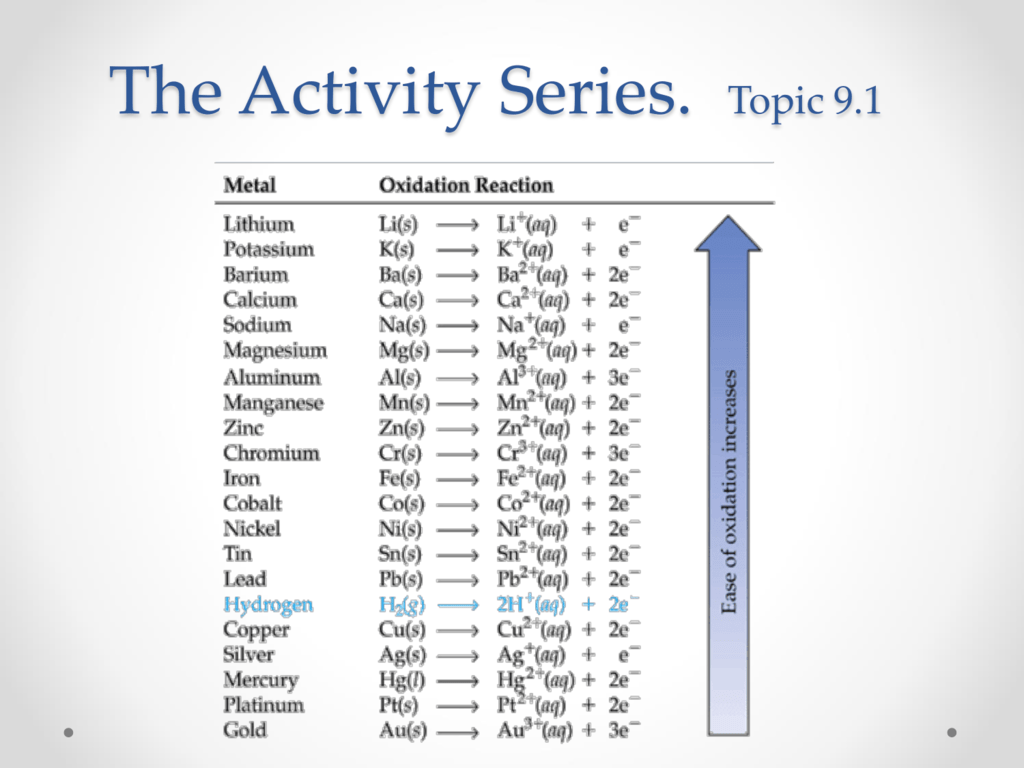 This picture illustrates activity series of metals essay.
This picture illustrates activity series of metals essay.
Reactivity series of metals
 This picture representes Reactivity series of metals.
This picture representes Reactivity series of metals.
Activity series worksheet
 This picture shows Activity series worksheet.
This picture shows Activity series worksheet.
Reactivity of metals
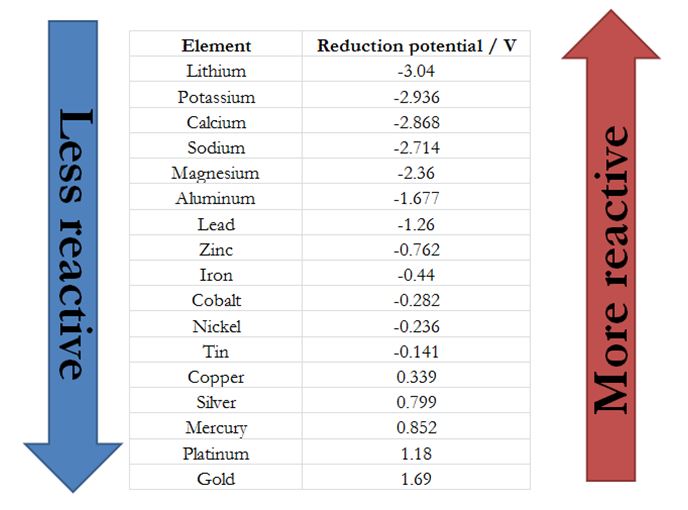 This image shows Reactivity of metals.
This image shows Reactivity of metals.
Activity series chemistry
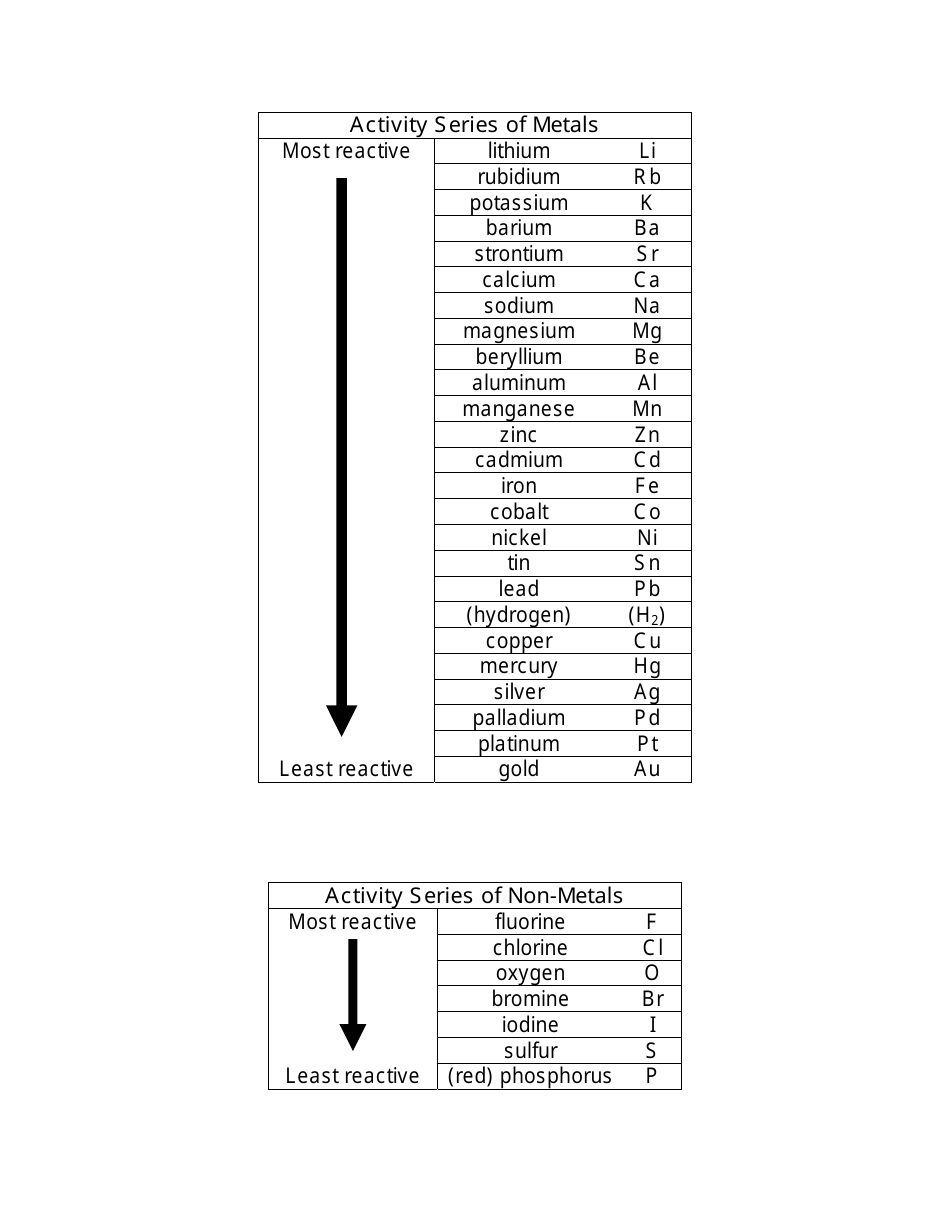 This picture representes Activity series chemistry.
This picture representes Activity series chemistry.
Lightest metal
 This picture shows Lightest metal.
This picture shows Lightest metal.
Properties of metals
 This image illustrates Properties of metals.
This image illustrates Properties of metals.
What is the activity series
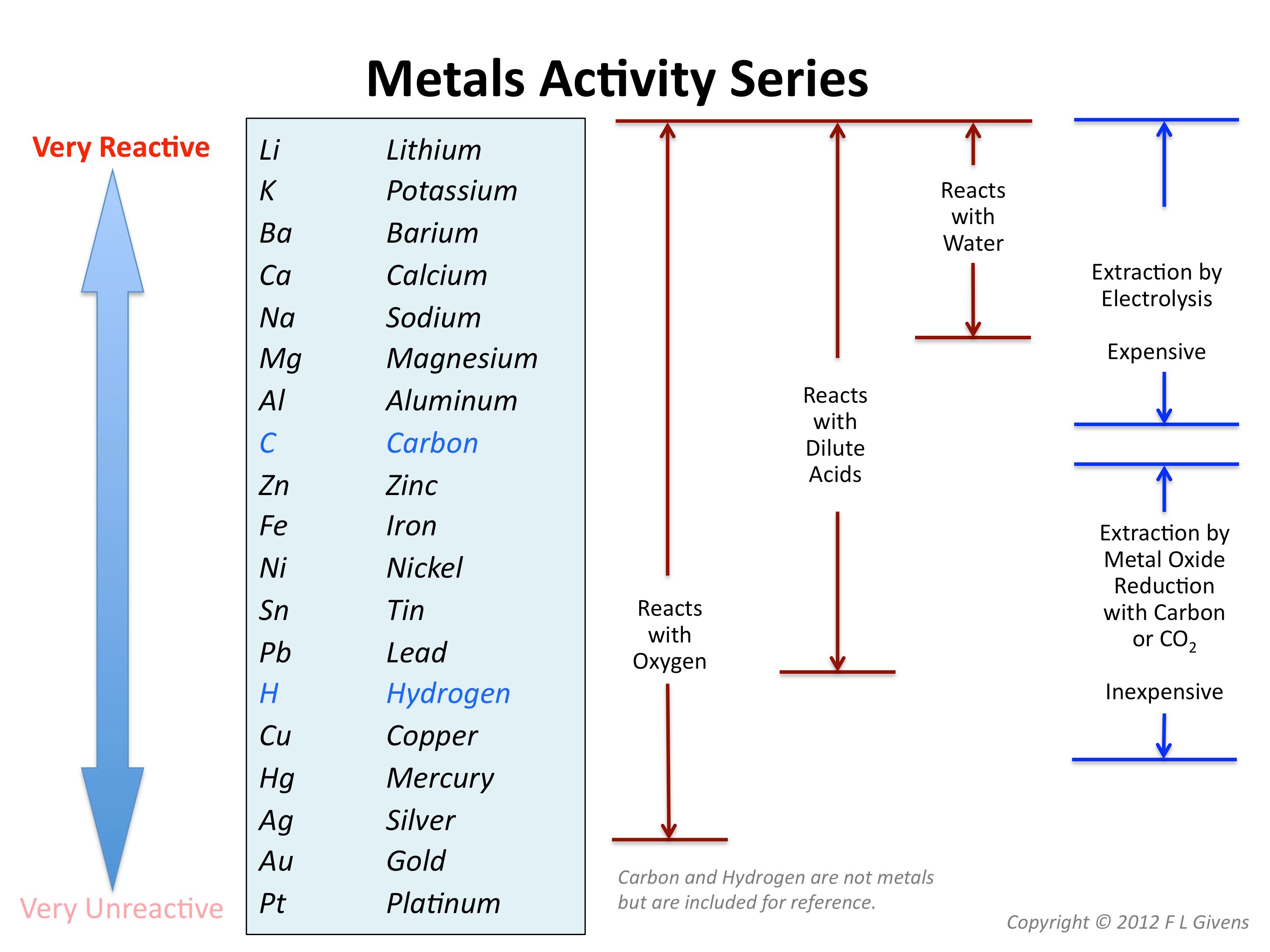 This image demonstrates What is the activity series.
This image demonstrates What is the activity series.
How to remember the reactivity series of metals?
The following table shows the reactivity series of metals and how to remember them using a mnemonic. Scroll down the page for examples and solutions. How metals react with water and with dilute acids and how to use this information to order the elements by reactivity.
How are metal displacement and activity series related?
Metal Displacement and the Activity Series. Metals differ in their tendency to lose electrons; more reactive metals lose electrons more easily. A more reactive metal is able to donate electrons to the ion of a less reactive metal in a displacement reaction.
How are the reactions between metals and acids predicted?
Thus, the reactions between metals and some acids can be predicted with the help of the reactivity series. The ions of low ranking metals are readily reduced by high ranking metals on the reactivity series. Therefore, low ranking metals are easily displaced by high ranking metals in the single displacement reactions between them.
Which is an application of the reactivity series?
Apart from providing insight into the properties and reactivities of the metals, the reactivity series has several other important applications. For example, the outcome of the reactions between metals and water, metals and acids, and single displacement reactions between metals can be predicted with the help of the activity series.
Last Update: Oct 2021
Leave a reply
Comments
Shantey
25.10.2021 02:59Metals react differently with different substances. This chemical science video tutorial explains how to learn if a only replacement reaction testament proceed as printed using the activenes series of metals.
Khari
25.10.2021 12:58A more reactive gold-bearing is able to donate electrons to the ion of a less labile metal in A displacement reaction.