Are you ready to discover 'how to write significant figures in scientific notation'? All the details can be found here.
Zeros after the denary point and aft figures are significant; in the turn , the 2, 4, 5 and last 0 ar significant. Exponential digits in scientific notational system are not significant; has three world-shaking digits, 1, 1, and 2. These rules ensure high-fidelity representation and interpreting of data.
Table of contents
- How to write significant figures in scientific notation in 2021
- Scientific notation answers
- Rounding significant figures
- Adding significant figures
- Scientific notation to 3 significant figures calculator
- 2 significant figures calculator
- Scientific notation significant figures calculator
- How many significant figures in 1000
How to write significant figures in scientific notation in 2021
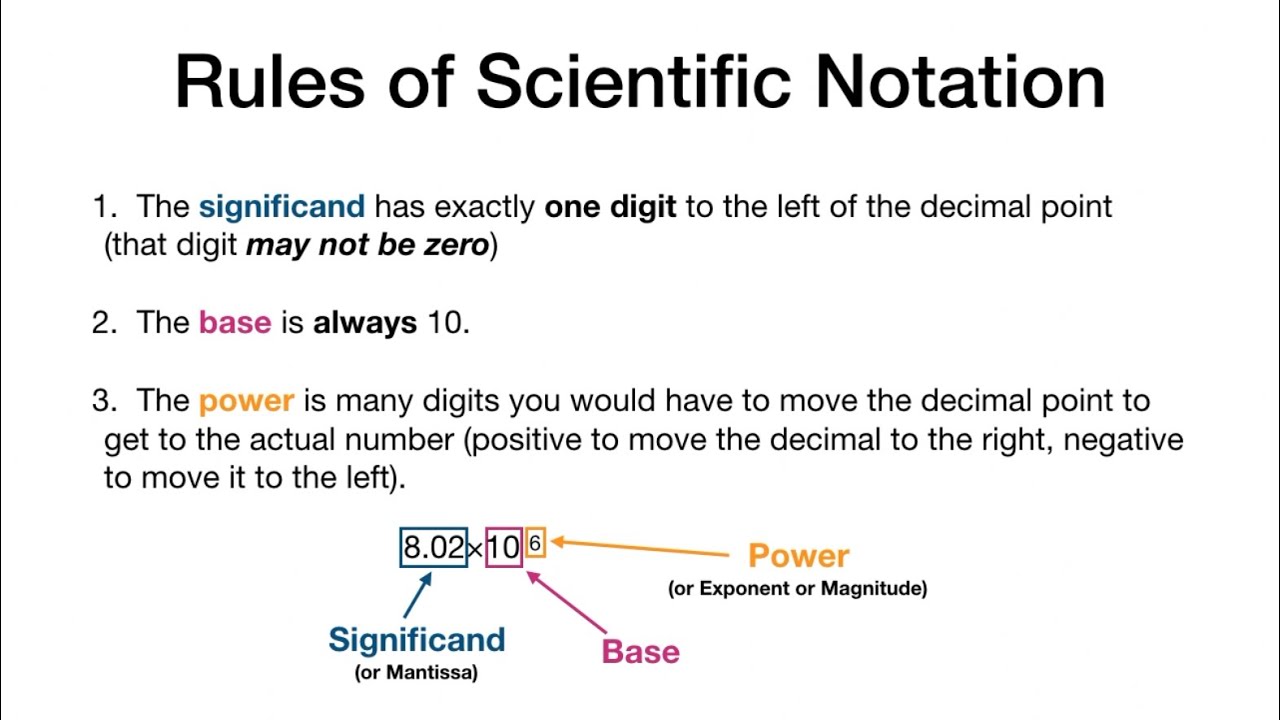 This image demonstrates how to write significant figures in scientific notation.
This image demonstrates how to write significant figures in scientific notation.
Scientific notation answers
 This image demonstrates Scientific notation answers.
This image demonstrates Scientific notation answers.
Rounding significant figures
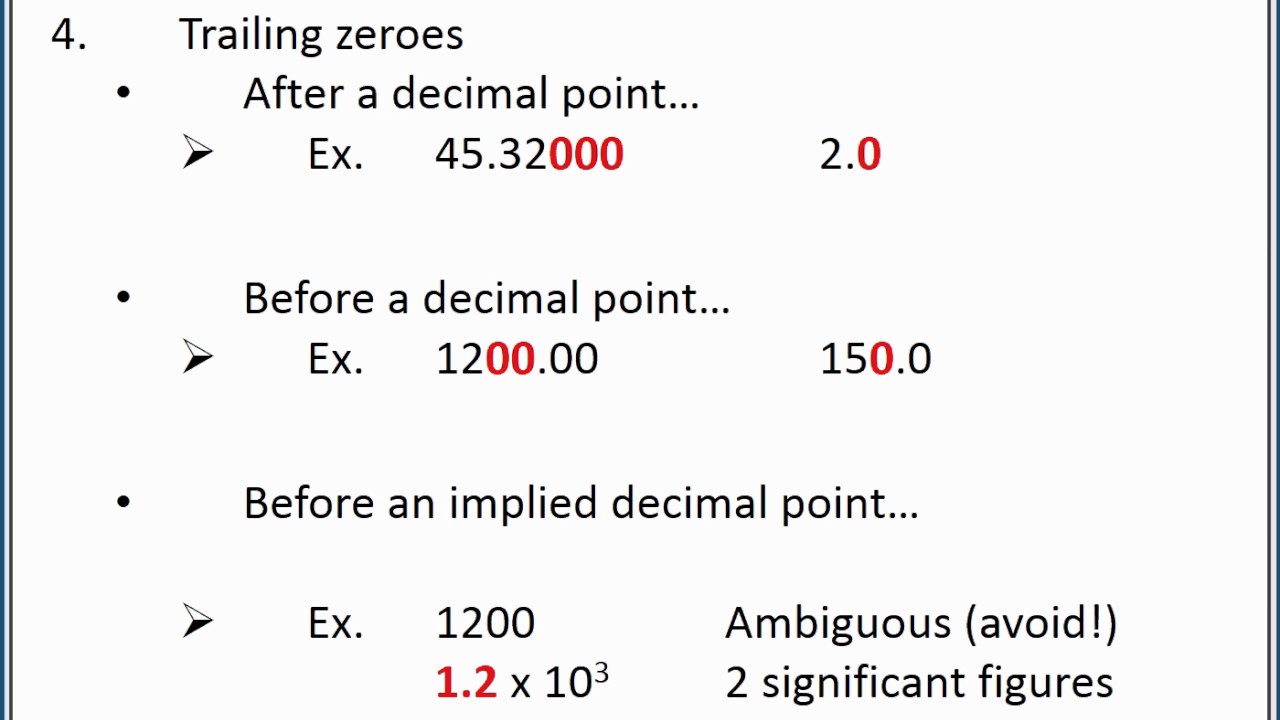 This image representes Rounding significant figures.
This image representes Rounding significant figures.
Adding significant figures
 This picture representes Adding significant figures.
This picture representes Adding significant figures.
Scientific notation to 3 significant figures calculator
 This image representes Scientific notation to 3 significant figures calculator.
This image representes Scientific notation to 3 significant figures calculator.
2 significant figures calculator
 This image representes 2 significant figures calculator.
This image representes 2 significant figures calculator.
Scientific notation significant figures calculator
 This picture representes Scientific notation significant figures calculator.
This picture representes Scientific notation significant figures calculator.
How many significant figures in 1000
 This image representes How many significant figures in 1000.
This image representes How many significant figures in 1000.
How to add and subtract numbers in scientific notation?
Multiply the decimal number by 10 raised to the power indicated. See the Scientific Notation Calculator to add, subtract, multiply and divide numbers in scientific notation or E notation. To round significant figures use the Significant Figures Calculator .
How are significant figures determined in scientific notation?
In addition and subtraction the number of significant figures that can be reported are based on the number of digits in the least precise number given. Specifically this means the number of digits after the decimal determine the number of digits that can be expressed in the answer.
When do you use zeros in scientific notation?
Note: Zeros used to show where a decimal point belongs are not significant. Rules to ensure that your answers always contain the correct number of significant figures: In multiplication and division, the answer must contain the same number of significant figures as the term with the least number of significant figures.
Do you report the correct number of significant figures?
Rules to ensure that your answers always contain the correct number of significant figures: In multiplication and division, the answer must contain the same number of significant figures as the term with the least number of significant figures. Example: (16.79) (14.6) = 245.134. The answers should be reported as 245.00. Why?
Last Update: Oct 2021